Abstract
Background:
RI is one of the most common complications of multiple myeloma (MM). The incidence of RI at diagnosis ranges from 20% to 50%, Bortezomib-based regimens remain the cornerstone of the management of myeloma-related RI, and the addition of a third drug to Vd seems to be beneficial,but not for sure. Pomalidomide is extensively metabolized by liver, with only approximately 2% of active substance eliminated in the urine, which suggests that RI may not affect pomalidomide exposure in a clinically relevant manner. The MM013(phase 2, NCT02045017) trial confirmed that Pomalidomide could be safely used in RRMM with RI and achieved good response in MM and Renal function. The OPTIMISMM (phase 3, NCT01734928) trial Subgroup analysis reported efficacy and safety of PVd vs Vd at first relapse by renal function (creatinine clearance [CrCl] < 60 vs ≥ 60 mL/min) before. The ORR significantly improved with PVd vs Vd regardless of renal status (P < .001 for both renal groups): 91.4% vs 53.6% (≥ VGPR,54.3% vs 21.4%) in the CrCl < 60 mL/min group and 89.5% vs 55.2% (≥ VGPR, 64.5% vs 23.0%) in the CrCl ≥ 60 mL/min group. Since RVd is the standard treatment for NDMM patients, We believe that standard dose Pomalidomide plus Vd will be very promising for NDMM patients with RI
Study Design/ Methods:
This is a multicenter, prospective, randomized phase 2 study designed to evaluate the efficacy and safety of PVd Versus Vd in Patients With NDMM and Renal Impairment(RI).
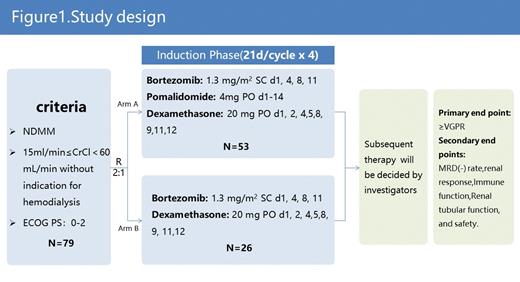
Eligible patients were aged ≥18 years and had a diagnosis of multiple myeloma, measurable disease, and an Eastern Cooperative Oncology Group performance status of 0-2. Patients were required to have had no prior antimyeloma regimen. The definition of RI is based on reduced creatinine clearance (15 mL/min≤CrCl<60 mL/min) without indication for hemodialysis, which have to be the result of myeloma. Key exclusion criteria included previous course of chemotherapy; uncontrolled malignant disorder, infection, or peripheral neuropathy, patients on dialysis will be excluded too.
Approximately 79 patients will be randomized 2:1(Figure 1) to Arm A (PVd) or Arm B (Vd), both Arms will have induction therapy for 4 cycles. In Arm A, patients will receive pomalidomide 4 mg on days 1-14 of each cycle. Bortezomib 1.3 mg/m 2 on days 1, 4, 8, and 11 of each cycle. Dexamethasone 20mg on days 1, 2, 4, 5, 8, 9, 11, and 12 of each cycle (21-day cycles); In Arm B, patients will receive the same bortezomib and dexamethasone as Arm A.
The primary endpoint is ≥VGPR, the secondary endpoints are MRD(-) rate,renal response,Immune function,Renal tubular function, and safety. Myeloma response and renal response will be assessed by the International Myeloma Working Group criteria after each cycle. Eficacy analyses will be performed on the intent-to-treat population; Safety analysis will be conducted in the safety population, which are composed of all patients who received ≥1 dose of study medication. The trial is currently enrolling and will be open in 8 sites in China.
Disclosure:Research Sponsor : CHIATAI TIANQING PHARMACEUTICAL GROUP.
No relevant conflicts of interest to declare.
Pomalidomide which was approved in RRMM will be used in NDMM with RI